What is a thermal battery?
Thermal mass of any kind can by definition be called a thermal battery, as it has the ability to store heat. In the context of a house, that means dense materials like bricks, masonry and concrete. Even a jug of water sitting in a sunny window is a thermal battery of sorts as it captures and later releases heat from the sun.
A well-insulated concrete floor acts as a thermal battery as well; once you pump it full of heat it takes a long time to cool down (depending on the thickness), and it regulates interior temperatures during that time.
One practical use for getting the most from a radiant concrete floor as a thermal battery would be in areas with fluctuating energy costs - you can set your floor on a timer so it only comes on during low-rate hours (7pm to 7am in Ontario for example). During the twelve hours that it is off, it acts as a battery by slowly releasing the stored heat, so you avoid having to pay the higher rates during peak-hours.

As you move into the area of active heat-storage systems, one of the more common types of thermal battery (not that there are a lot of them) is a huge water tank buried in the ground that is heated by solar thermal panels.
Even this type of system is not new, the first house in the United States with an active solar heating system was built In 1939 on the MIT campus (Massachusetts Institute of Technology), and sat on top of a huge water reservoir that was heated by thermal solar panels.

What are phase change thermal batteries?
Taking advantage of a 'phase change' raises the bar a bit - stick with me, it will be fun, I promise :)
A significant input of energy is required to cause a material to change from a solid to liquid. That energy is later released when that material solidifies again. While those transformations are happening and the material is either absorbing or releasing energy, the temperature will stay constant. Once the phase change is complete, the material will begin to change temperature again.
So what does that mean in real terms? It means that in order to melt water, wax, metal, rock or whatever, you need to feed it a ton of energy. but the temperature doesn’t change while you are doing that. So your ‘battery’ has more power, and you can store more heat in the same volume of space.
It’s difficult to take advantage of a melting point of 0° Celsius, but wax melts at about 37° Celsius (depending on its exact chemical makeup), which is perfect for collecting and storing heat from solar thermal collectors.

How to build a thermal battery:
If you had a heat-collecting solar panel (directly heating air or liquid rather than generating power with photovoltaics), you can use that to charge your thermal battery. Envision this - a large tank of wax (or water) that is warmed by heated coils from a solar collector. Through that same tank runs another coil that is extracting the heat to pump it through your radiant floor or whatever other heating distribution system you have.
Specific Heat Capacity:
If you take solid paraffin (heat capacity Cp = 2.5 kJ/kg·K and heat of fusion of 210 kJ/kg), let's say 1 kg, at room temperature, you will need 2.5 kJ (kilojoules) of heat to make the 1 kg block go from 20°C to 21°C. To make it go from 21°C to 22°C, you will also need 2.5 kJ (i.e. the same amount of energy).
Paraffin melts at approximately 37°C. If it drops to 36°C, you will again only need 2.5 kJ to bring it back to 37°C, but you would need 210 kJ (84 times as much) to go from 37 to 38°C.
This is because in order to melt, some chemical bonds in the solid lattice need to be broken, a process that requires extra energy. So overall, if a kg of paraffin is lying around at 20°C, you would need 252.5 kJ to bring it to 38°C.
One of the more common building materials with thermal mass benefits is concrete. In contrast to paraffin, 1 kg of concrete (Cp = 0.88 kJ/kg·K) would need 15.8 kJ to do the same. For water (Cp = 4.18 kJ/kg·K), the amount of energy required would be 75.2 kJ.
The amount of energy put in is the amount of energy stored in a material, as this energy will later be released as the material cools back down to 20°C, or room temperature. While there are many materials that can be used in the application of heat storage, this is just a quick comparison of some of the more commonly available ones.
So to conclude, paraffin can store 16 times as much heat per kg as concrete, and 3.4 times as much as water. So while water may not be the best material to store heat, it certainly is the most affordably priced and easily accessible.
See more about Passive Solar Home Design here
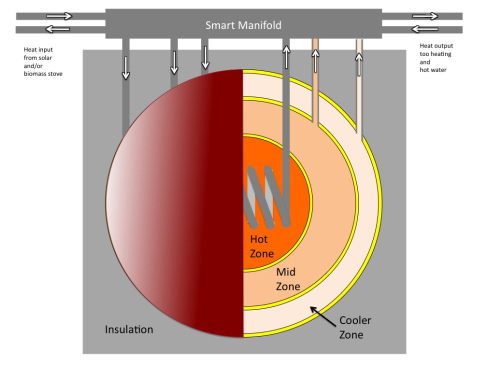 |
|
Thermal battery diagram courtesy of alternative-photonics.com/
|
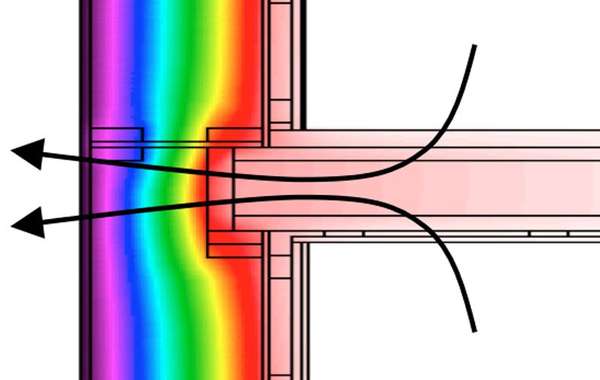
Thermal battery diagrams are courtesy of Alternative Photonics.


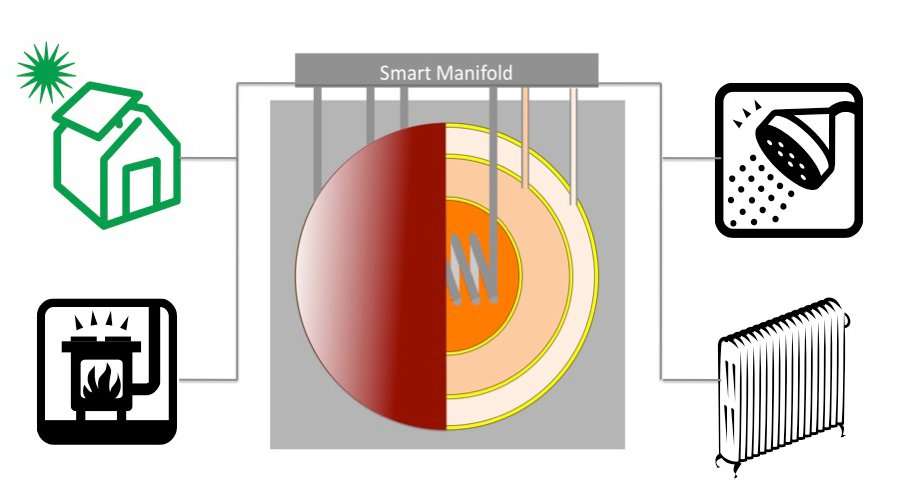




























In the 80's I built a solar home in Nebraska which used "thermal batteries". It was super tight , had 16 patio door size windows and had only a wood stove for heat. We averaged one cord of wood a year - in winter cloudy Nebraska.
The heat from the glass wall rose to the roof and was sucked down a concrete block wall into a basement closet filled with hundreds of water jugs. The water slowly released the heat overnight.
We insulated the glass wall at night with a rollup rigid R8 insulated shade called the Insulider.
The plastic bottles you are burying make terrific "thermal batteries".
That's pretty impressive Jerry, and a pretty brilliant design to collect and store heat.
My shed /dog house has heat by a passive solar heater I figure it produce 15 to20 BTU daily. I wanted to store so I did the candle wax the one that goes in jars .I figure it 120000 btu of store heat. It will last about three day with no sun the ceiling heat coming in at 137 degrees F. So the top part of the shed feel like a sauna and the bottom is about 75 to 80F and the dogs love it.
Hi Gerald,
That is brilliant! When I built a dog house I put a 'facing window that I figured with give him additional comfort on a cold day, but he unfortunately had no interest in ever going in it. (Free passive solar dog house for pickup never been used :) )
What you have actually done is give your dogs a phase change thermal battery for storing heat, in case you didn't see that article. We think / hope that phase change building materials may be encorporated into energy-efficient home design in the future to help balance temperatures and create a more comfortable indoor environment for humans.
I'm late to this discussion, hopefully not too late. Could a phase change thermal battery be coupled with PV and a heat pump for time shifting ? My notion is to heat the wax during the day when the sun is shining and ambient temps are high enough to allow efficient heat pump operation, and then release the heat after sunset.
Thank you for posting this.
The notion of leveraging a material's phase change as one of a thermal battery's key aspects could really optimize a design, due to elimination of wild temperature swings.
Yet, if one were to design a series of batteries with differing materials to provide a graduation of phase change temperatures, it seems perhaps higher energy densities could be achieved.
I really like the ideas you've stocked in me. Thank you.
That's really nice feedback to hear, thanks for taking the time to share that.